Cyclodextrins (CDs) are a family of very unusual cyclic molecules composed of a-1,4-linked D-glucose repeat units. They are made up of six or more α-D-glucopyranoside units, as in amylose (a component of starch). Their cyclic structure creates a cone shape molecule: for α (alpha)-Cyclodextrin it is comprised of six Glucose subunits, for β (beta)-Cyclodextrin it is seven Glucose subunits and for γ (gamma)-Cyclodextrin it is eight Glucose subunits. Cyclodextrins have thus characteristic toroidal shapes, with the smaller opening of the toroid (primary rim) representing the C6-OH primary hydroxyls and the larger opening (secondary rim) the C2-OH and C3-OH secondary hydroxyls. The inner part of the toroid provides a hydrophobic cavity that can accommodate various organic guest molecules.
Their most unique structural feature is responsible for their ability to reversibly form inclusion complexes by encapsulating guest molecules within their hydrophobic cavities. Complexes can be formed with a wide variety of solid, liquid and gaseous compounds. The size of respective Cyclodextrins controls the size of the guest molecules they can host, with molecules exceeding the CD cavity size typically being excluded from complexation. In some cases, however, two CDs are even known combine to complex a single, lager size guest molecule from opposite directions. Thus, the antispasmodic Alverine citrate can form 2:1 inclusion complexes with α and β-Cyclodextrins. The resulting Alverine citrate complexes display enhanced drug bioavailability.
The CDs have different cavity diameters based on the number of Glucose units in their structure. The cavity diameter is a critical parameter for inclusion complex formation because the guest molecule must fit into the CDs cavity based on their geometry and size comparable to the “lock and key” principle. Furthermore, the guests must be hydrophobic due to the hydrophobic cavity of the CD. The more hydrophobic the guest is, the stronger the interaction between guest and the CD host. The interactions between the host and the guest molecules are noncovalent: e.g., ion–dipole, hydrogen-bonding and van der Waals types.
Since the core of the Cyclodextrins is hollow it offers a means of including molecules of appropriate size. The water soluble Cyclodextrin portion of such complexes can serve to “solubilize” lipophilic molecules, such as drugs. This can lead to enhancing drug bioavailability and the solubility of poorly water-soluble materials, such as lipophilic drugs. Cyclodextrins can thereby mask or deliver odor and taste of guests, or reduce drug side effects. Even certain polymers, e.g., poly(ethylene oxide)s, can form complexes with cyclodextrins. In addition, Cyclodextrin nanosponges and nanofibers are also reported.
The following graphics and table is from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10534465/
Without addressing copyright issues:
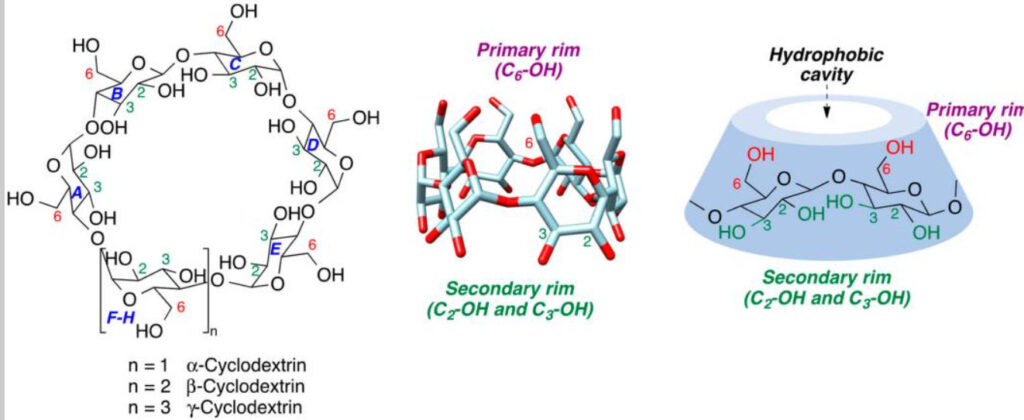
The general structure of the natural Cyclodextrins. The glucopyranose rings are identified with the letters A–H. Positions 2, 3, and 6 bear hydroxyl groups. The secondary hydroxyls (C2 and C3) are oriented toward the broader rim of the Cyclodextrin cavity and the primary hydroxyls (C6) are oriented toward the narrower rim.
Table 1
Physicochemical properties of natural Cyclodextrins.
| CD | Cavity Diameter (Å) | Outer Diameter (Å) | Cavity Volume (Å3) | Height (Å) | Molecular Weight (g/mol) | Aqueous Solubility (mg/mL) |
| α | 4.7–5.3 | 14.6 | 174 | 7.9 | 973 | 145 |
| β | 6.0–6.5 | 15.4 | 262 | 7.9 | 1135 | 18.5 |
| γ | 7.5–8.3 | 17.5 | 427 | 7.9 | 1297 | 232 |
WHAT IS THE SOURCE OF CYCLODEXTRINS?
Cyclodextrins occur naturally and are manufactured in large scale from starch by enzymatic conversion with Cyclodextrin glycosyltransferase (CGTase) along with α-amylase. One manufacturing method uses dedicated enzymes, that can specifically produce α-, β- or γ-cyclodextrins. Purification of the CD products can be accomplished based on the differential water solubility and crystallization of the CDs or alternatively by more expensive processes of complexation.
WHAT IS THE USE OF CYCLODEXTRINS?
Due to their biocompatibility, biodegradability, and relatively low-cost CDs have a broad range of uses. Cyclodextrins can play an important role in food, pharmaceutical, drug delivery, and chemical industries, as well as in agriculture and environmental engineering. Cyclodextrins and their derivatives are functional excipients that can improve the bioavailability of numerous drugs. Because of their drug solubility improving properties they are widely used in many pharmaceutical products.
Their applications have also been extended to the recovery of soil and water owing to their capacity to capture pollutants in their cavities.
Pharmaceutical grade CDs have been approved for oral, parenteral and nasal use. In nearly all formulations intended for any route, the basic function of Cyclodextrins is to manage the solubility, confer greater bioavailability and solubility of the complexed guest agent. Many other critical properties can also be modified by CDs. CDs are used in many cosmetic and personal care products.

HOW ARE CYCLODEXTRIN COMPLEXES FORMED?
Cyclodextrins readily form complexes. Cyclodextrin complexes can be prepared in different ways, including by physical mixing, co-precipitation, kneading, super critical carbon dioxide, grinding, microwave irradiation, solvent evaporation, spray-drying and freeze drying.
EVIDENCE FOR COMPLEXATION
The fact that Cyclodextrins form inclusion complexes can be demonstrated by a number of analytical techniques, such as by NMR (Nuclear Magnetic Resonance), UV–visible, FT-IR, differential scanning calorimetry and X-ray spectroscopy and by differences in various chemical and physical properties.
BENEFITS OF COMPLEXATION
What are the advantages of Cyclodextrin inclusion complexes?
Cyclodextrin complexes can improve and significantly alter the chemical, biological, stability and physical properties of many substances, including drugs, food, and other compounds.
As mentioned, Cyclodextrin complexes are used for the enhancement of various physicochemical properties (such as solubility, bioavailability, taste masking, permeability, and stability) of drugs, food ingredients and other products.
Drugs
CDs can increase drug solubility and potency, which can reduce drug toxicity and irritation. For example, β-CD can increase the antiviral activity of ganciclovir, which can reduce its toxicity. CDs can also reduce the toxicities associated with poorly water-soluble drugs that crystallize in parenteral formulations. The mechanism of controlled degradation of such complexes is often based on changing the pH of water solutions, leading to the loss of hydrogen or ionic bonds between the host and the guest molecules. Alternative means for the disruption of the complexes take advantage of heating or the action of enzymes able to cleave α-1,4 linkages between Glucose monomers. Cyclodextrins can also enhance mucosal penetration of drugs.
Food Ingredients
CDs can improve the solubility, stability, and elimination of food compounds from the body. For example, Lemon essential oil β-CD (LEO@β-CD) can delay the decay and color deterioration of blackberries when stored at different temperatures. CDs can be employed to emulsify mixtures. CDs can also be used to mask odors of food ingredients, such as that of garlic. Cyclodextrins bind fragrances. Such devices are capable of releasing fragrances when heated, such as by ironing, body heat, or a dryer. A common application is a typical ‘dryer sheet’.
Cyclodextrins are also used to produce alcohol powder by encapsulating Ethanol. The powder produces an alcoholic beverage when mixed with water, or it can also be manufactured in tablets. CDs can be used in the entrapment of essential oils, such as Cinnamon oil.
Other Products
CDs can improve the solubility of anticancer flavonoids, such as curcumin and quercetin, which are poorly soluble in water. A CD complex of curcumin has also shown superior antiproliferative and anti-inflammatory activities compared to free curcumin.
CYCLODEXTRIN DERIVATIVES
Interest in Cyclodextrin derivatives is also enhanced because their host–guest behavior can be manipulated by chemical modification of their hydroxyl groups. The full spectrum of standard carbohydrate modification methods can be employed for Cyclodextrins.
The Cyclodextrins can be selectively modified at a single Glucose unit (as for example in 2-O-Mono-(2-hydroxy)-propyl-a-cyclodextrin), disubstituted (as for example in 6A,6B-Diiodo-6A,6B-dideoxy-b-cyclodextrin) or at all Glucose units (as for example in Octakis-(2,3,6-tri-O-acetyl)-g-cyclodextrin), as well as at their primary (CH2OH) or secondary (CHOH) alcohol functions. Such modifications then afford products which are either mono-, di-, or fully substituted with various resulting properties. The degrees of derivatization are readily adjustable. Cyclodextrins can also be randomly modified. A very large array of Cyclodextrin derivatives (over 1,500) have so far been reported.
O-Methylation and acetylation are some typical conversions that afford Ethyl, Butyl and Methyl Cyclodextrins. Carboxyethyl, Carboxymethyl and Hydroxyethyl Cyclodextrins. Modification with Propylene oxide affords hydroxypropylated derivatives. Cyclodextrin sulfates and phosphates have been reported among many other derivatives. The attachment of thiol groups to CDs confers high mucoadhesive properties and the resulting thiolated products are capable of forming disulfide bonds with cysteine-rich mucus glycoproteins. The gastrointestinal and ocular residence time of thiolated Cyclodextrins can thereby be substantially prolonged.
Cyclodextrins can also be converted to polymers with varying properties. CDs with pendent polymers can serve as hosts to encapsulate many drug molecules that otherwise cannot be readily complexed by free Cyclodextrins. Thus, polymeric nanoparticles have been prepared by a host–guest interaction between a prodrug of the anticancer drugs camptothecin (CPT) and naphthalimide (NAP) containing an adamantane molecule (AD) and a CD-pendent hyaluronic acid (HA) polymer, which endows the assembly with colloidal stability and biocompatibility.
ARE CYCLODEXTRINS SAFE?
Native CDs can be ingested without significant absorption, being thus ‘Generally Regarded As Safe’ (GRAS) by the FDA. Cyclodextrins consumed orally in food or otherwise do not usually enter the circulation in significant amounts and are thus generally safe. Cyclodextrins are key ingredients in more than 30 different approved medicines.
The safety profiles of native cyclodextrins are well established. They are accepted as food additives and GRAS in the United States. HPβCD is listed in the US, EP and JP Pharmacopoeias. The approval of Cyclodextrins and the acceptable complex concentrations may vary between territories or regions. Also, not all Cyclodextrins are recommended for all routes of administration. For example, both α-CD and β-CD show renal toxicity after parenteral administration and are thus generally not recommended for medicinal products administered intravenously.
Most of cytotoxic chemotherapeutic agents have poor aqueous solubility. These molecules are associated with poor physicochemical and biopharmaceutical properties, which makes their formulation difficult. The systems based on Cyclodextrin complexation and nanotechnology can camouflage the undesirable properties of drug and lead to synergistic or additive effects. Cyclodextrin-based nanotechnology can provide a better therapeutic effect.
REGULATORY STATUS OF CYCLODEXTRINS
In Japan, native CDs are regarded as natural products and are therefore used without many restrictions both in medicines and in foods. In western countries, the ingestion of native cyclodextrins is regulated by the JECFA (Joint WHO/FAO Expert Committee on Food Additives) with the pharmaceutical applications falling under the European Medicines Agency (EMA) in Europe and under the Food and Drug Administration in the United States of America (FDA). Native CDs can be ingested without significant absorption, being rated as GRAS by the FDA. α-CD and γ-CD can be taken without restrictions, while the oral intake of β-CD should not exceed 5 mg per kilogram of weight per day. Native cyclodextrins suffer from much stronger restrictions for parenteral use. EMA recommends against the administration of α-CD and β-CD directly into the bloodstream due to renal toxicity. Native CDs are also known to cause hemolysis in vitro.
Only relatively few pharmaceutical Cyclodextrin products and complexes are so far approved for human use. The FDA has approved 2-Hydroxypropyl-β-Cyclodextrin (HPβCD) and 2-Hydroxypropyl-γ-Cyclodextrin (HPγCD) as inert excipients, with HPβCD being suited for oral and intravenous administration, while HPγCD can only be used in topical products. Heptakis-2,3,6-tris-O-methyl β-Cyclodextrin (TRIMEB) is rated unsafe for human use due to its hemolytic action and renal toxicity. Heptakis-2,6-di-O-methyl-β- Cyclodextrin (DIMEB) also features some toxicity.
Cyclodextrins that have undergone O-methylation in random positions with different degrees of substitution display different safety profiles. RAMEB (randomly methylated beta-Cyclodextrin) with an average of 1.8 methoxyl groups per Glucose unit has some hydrolytical effects on erythrocytes and renal toxicity. EMA therefore does not permit RAMEB for parenteral use. CRYSMEB (Crystalline Solid, Crystalline Methylated β-Cyclodextrin) has a deliberately low substitution degree (0.56 methyl groups per Glucose unit). It was designed for high biotolerability. CRYSMEB does not cause hemolysis and is approved for dermal uses and in cosmetics. Another biocompatible CD is Sulfobutyl ether β-CD (SBEβCD), developed to be non-nephrotoxic and employed in several marketed medications for both oral and intravenous administration.
WHAT ARE THE DISADVANTAGES OF CYCLODEXTRINS?
In spite of their countless advantages, Cyclodextrins also have some limitations. For example, when β-Cyclodextrin interacts with cholesterol it forms a low soluble complex that may have a nephrotoxic impact. Oral 200 mg/kg/day Cyclodextrins may cause digestive problems such as diarrhoea. At high doses CDs can cause reversible diarrhoea and cecal enlargement in animals. Parenteral 200 mg/kg/day use for over extended periods may cause kidney disease.

CARBOMER, INC.’S CYCLODEXTRIN PRODUCTS AND CYCLODEXTRIN COMPLEXES
CarboMer, Inc.’s CD products stand out due to their high-quality composition. CarboMer, Inc. offers a range of CD products for R&D and pharmaceutical applications. They are available as solids and solutions and with different molecular weight ranges, MS and substitution levels.
CarboMer, Inc.’s CD products stand out due to their consistent high-quality. CarboMer, Inc. offers a substantial range of CD products for R&D and pharmaceutical applications. Among the available products are Cyclodextrin analytical standards, monosubstituted disubstituted and heterosubstituted Cyclodextrins, carboxyalkylated and hydroxyalkylated CDs, such as Hydroxypropyl-b-cyclodextrin.
Other CarboMer, Inc. products include Nitroxide-labelled derivatives, e.g.,Cyclotempo A™ [4-N-(4-amino-2,2,6,6-tetramethylpiperidine-1-oxyl)] a-cyclodextrin, a water soluble a-cyclodextrin, as well as many fluorescently labelled CDs, such as Cyclodans™ 12, a Dansyla-cyclodextrin water soluble, anionic a-cyclodextrin derivative.
Among other CD products are cationic derivatives, such as CatiMer™ BDN,N-Diethyl aminoethyl (DEAE)-b-cyclodextrin, tertiary amine b-Cyclodextrin suitable for gene delivery, Cyclodextrin Phosphates, Cyclodextrin Succinates, Cyclodextrin Sulfates, CarboMer, Inc. offers many Cyclodextrin polymers, such as b-Cyclodextrin polymer, Carboxymethyl a-cyclodextrin polymer and b-Cyclodextrin sulfate polymer.
CarboMer, Inc. offers many pharmaceutical grade and multi-compendial compliant CDs, e.g., b-Cyclodextrin USP (Betadex) Hydroxypropyl-b-cyclodextrin USP, Methyl b-cyclodextrin USP and Sulfopropyl-b-cyclodextrin (Betadex Sulfobutyl Ether Sodium).
CarboMer, Inc. also offers numerous Cyclodextrin complexes with a wide range of guest molecules, such as Cholesterol, Diazepam and garlic oil. In addition, complexes are available with CD derivatives, such as Methyl-b-Cyclodextrin and Hydroxypropyl-b-Cyclodextrin.
Furthermore, unlike other manufacturers, CarboMer, Inc. prepares on a small or large scale and on a customized basis CD derivatives and complexes to specific client requirements.
COMPOSITION AND SOURCE
CarboMer, Inc.’s offers a broad spectrum of high-quality, pharmaceutically relevant CD products with different composition and substitution patterns.
CarboMer, Inc. adheres to strict guidelines set by the Food and Drug Administration (FDA), ensuring the safety and quality of its CD products. Additionally, compliance with USP standards and those of other international regulatory bodies reflects CarboMer, Inc.’s commitment to providing the highest-quality CD products. Adherence to these regulatory standards guarantees that clients always receive reliable CD products of excellent quality.
SUMMARY
Cyclodextrins are important products that offer a wide range of medical benefits. However, it’s essential to be mindful of the potential risks and side effects, and to consult a healthcare provider when CD products uses are being considered. By understanding the benefits and safety considerations of CD products, you can make informed decisions to enhance their use.